Introduction
Anti CD19 CAR T-cell therapy is a breakthrough immunotherapeutic approach for relapsed, refractory B-cell malignancies. Despite initial excitement, long term progression free survival with single antigen targeted CD19 CAR T-cells range from 30-40% in aggressive B-cell NHL. Loss of target due to selective pressure against CD19 can lead to antigen downregulation and development of a CD19-negative clonal population. To overcome this resistance mechanism, we recently completed a Phase 1 anti-CD19, anti-CD20 (LV20.19) CAR T-cell trial demonstrating the safety of 2.5x10e6 cells/kg dose in patients with relapsed, refractory B-cell lymphomas (in press in Nature Medicine). We report below single cell cytokine studies from final LV20.19 CAR T-cell products using the Isoplexis single-cell proteomics device.
Methods
LV20.19 CAR T-cells were manufactured onsite using the CliniMACS Prodigy device on a fixed 14-day manufacturing platform with IL-2 for cell expansion (NCT03019055). Isoplexis cytokine analysis was limited to the cohort of patients treated at goal dose of 2.5x10e6 cells/kg (n=16). Leftover LV20.19 CAR T-cells from the original product were thawed, CD4 and CD8 T cells were enriched by immunomagnetic sorting, and the T-cell subsets separately stimulated with CD19-engineered K562 stimulators overnight. The stimulated cells were loaded onto single-cell, Adaptive Immune Isocode chips, and the chips read for 18 hours in an Isolight instrument to assess single-cell production of 32 individual cytokines. Isospeak software was used to analyze the single-cell data.
Results
15 patients had adequate samples for analysis. Results were stratified by response (complete responders (CR) versus partial responders (PR) or progressive disease (PD)). The Isoplexis device was utilized to generate a polyfunctional (i.e., ability to produce more that 1 cytokine) index score (PSI) that has been previously reported (Rossi et al. Blood 2018) to correlate with CAR T-cell treatment outcomes.
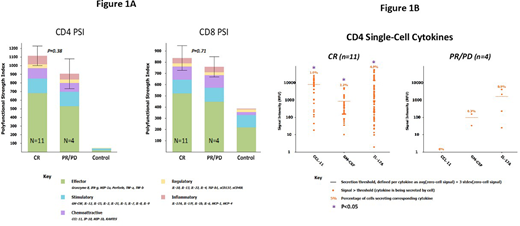
At Day 28 after LV20.19 CAR T-cell therapy, there were 11 CR patients and 4 PR/PD patients. CD4+ LV20.19 CAR T-cells from complete responders had a non-statistically significant decreased PSI score versus CD4+ cells from PR/PD patients (Figure 1A, p=0.38). The PSI score among CD8+ T-cells was similar (p=0.71) (Figure 1A). The increased PSI score among CR patients' CD4+ LV20.19 CAR T-cells was driven primarily by cells producing effector (IFNg and TNFb) cytokines. On a singular cytokine level, compared to PR/PD patient cells, CR patient CAR T-cells produced significantly higher levels of CCL-11, GM-CSF and IL-17A (Figure 1B; p<0.05). Notably, CR patients had dramatically higher percentages of individual CD4+ cells that secreted these 3 cytokines as compared to PR/PD patients (Figure 1B).
Conclusion
CD4+ LV20.19 CAR T-cells in responding patients trended towards a higher PSI than non-responding patients. At the single cell level, differences in the PSI among CD4+ LV20.19 CAR T-cells from responding patients were driven primarily by a few cytokines including IL-17A, CCL11, GM-CSF, IFNg, and TNFb, which may be key to clinical response. While our results are limited by sample size and only 4 PR/PD patients in this cohort, identification of cytokines correlating with response will allow selective modulation to improve clinical outcomes while limiting CRS. Ongoing studies with LV20.19 CAR T-cells will further delineate the role of IL-17A, GM-CSF and CCL-11 production as potential biomarkers of clinical response with bispecific CAR T-cell products.
Johnson:Cell Vault: Research Funding; Miltenyi Biotec: Research Funding. Fenske:Medical College of Wisconsin: Current Employment. Hamadani:Sanofi Genzyme, AstraZeneca: Speakers Bureau; Janssen R&D; Incyte Corporation; ADC Therapeutics; Celgene Corporation; Pharmacyclics, Omeros, AbGenomics, Verastem, TeneoBio: Consultancy; ADC Therapeutics: Membership on an entity's Board of Directors or advisory committees; Takeda Pharmaceutical Company; Spectrum Pharmaceuticals; Astellas Pharma: Research Funding. Dropulic:Lentigen, a Miltenyi Biotec Company: Current Employment, Patents & Royalties: CAR-T immunotherapy. Schneider:Lentigen, a Miltenyi Biotec Company: Current Employment, Patents & Royalties. Hari:GSK: Consultancy; Janssen: Consultancy; Takeda: Consultancy; Incyte Corporation: Consultancy; BMS: Consultancy; Amgen: Consultancy. Shah:Miltenyi Biotec: Honoraria, Research Funding; Celgene: Consultancy, Honoraria; Incyte: Consultancy; TG Therapeutics: Consultancy; Verastim: Consultancy; Lily: Consultancy, Honoraria; Cell Vault: Research Funding; Kite Pharma: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal